The Ideal Gas Law Is Equivalent to Charles's Law When
Create a graph of temperature and volume data. Rearrange the Ideal Gas Law so that the constant R is similarly equal to the variables.
The ideal gas law is equivalent to Charless law when.

. We do this by considering isothermal isobaric and isochoric thermodynamic processes. Rthe ideal gas constant. V T displaystyle Vpropto T So this means.
The ideal gas law is the final and most useful expression of the gas laws because it ties the amount of a gas moles to its pressure volume and temperature. No blocks are added to or removed from the lid. Which describes the proportionality that allows.
Memorize flashcards and build a practice test to quiz yourself before your exam. The Ideal Gas Law has more variables than Boyles Law and it contains the ideal gas constant R. The Ideal Gas Law is derived from a series of individual equations that relate two variables at a time.
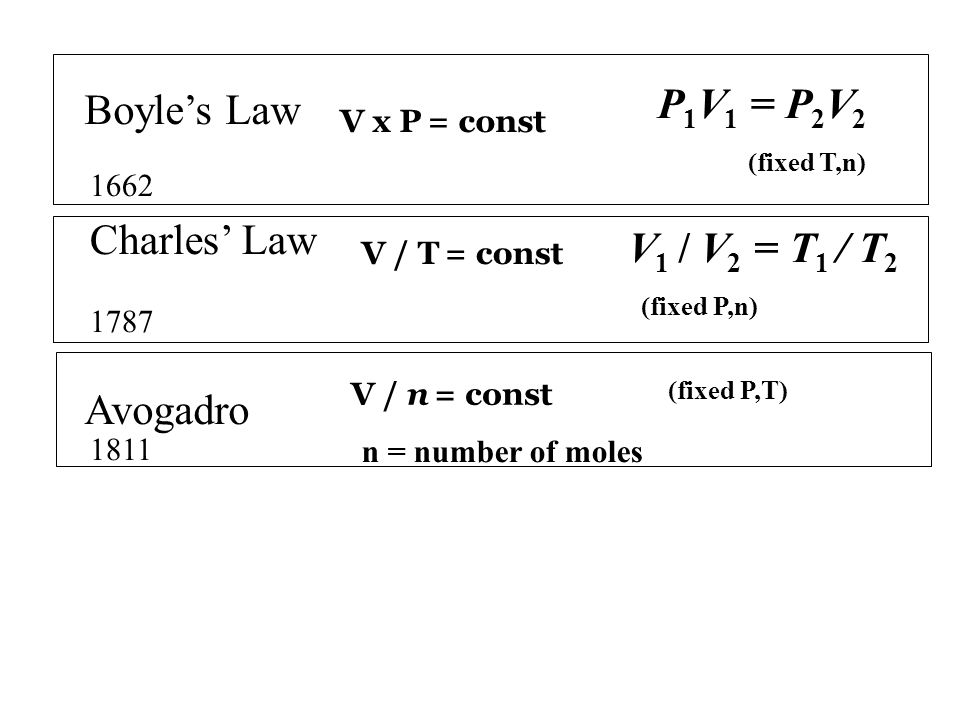
T temperature of the gas. The ideal gas law is equivalent to Boyles law when. We can use equation 6 to derive Boyles Charles and Gay-Lussacs Laws.
N denotes the number of moles. If any part is constant we can just isolate it and equal all of these to the other case. Temperature and Volume Data Syringe Volume mL 5 ml Temperature Conditions Temperature C Temperature K 29615 K Room Temperature 23 C 31815 K Hot Water 45 C 1 ml 27815 K Ice Water 5 C 03 ml Questions 1.
When the pressure on a sample of a dry gas is held constant the Kelvin temperature and the volume will be in direct proportion. What would be the V of the gas if its temperature were decreased to 100K. K constant which is equals to RTP where R is the universal gas constant T is temperature in Kelvin and P is the pressure.
Charless Law states that when the pressure is held constant the volume of a fixed mass of ideal gas is in direct proportion to the temperature in degrees Kelvin. V k T or V T k Where V is the volume of the gas T is the temperature in degrees Kelvin and k is a constant that depends on the pressure and amount of. The simplicity of this relationship is a big reason why we typically treat gases as ideal unless there is a good reason to do otherwise.
A modern statement of Charless law is. A combination of Boyles law and Charles law. Well the original equation is PV nRT.
The limitations are as follows. In this case the ideal gas law is also commonly written as. Similarly when we project on the temperature-volume plane we get Charless law T k V and for the temperature-pressure plane we have Gay-Lussacs law P k T.
R universal gas constant. So we have PV PV. Amedeo Avogadro in 1811 combined the conclusions of Daltons Atomic Theory and Gay Lussacs Law to give another important Gas law called the Avogadros Law.
General Gas Equation or Ideal Gas Law. Since the temperature and pressure is constant so RTP is also constant and represented as k. The individual laws are Boyles Law Charless Law Avogadros Law and Amontons Law.
It is a good approximation of real gases under low pressure andor high temperature. The ideal gas law is equivalent to Avogadros law when. Charless Law can be written mathematically as follows.
For example the R is often constant so we can set up. V T k or V. P pressure of the gas.
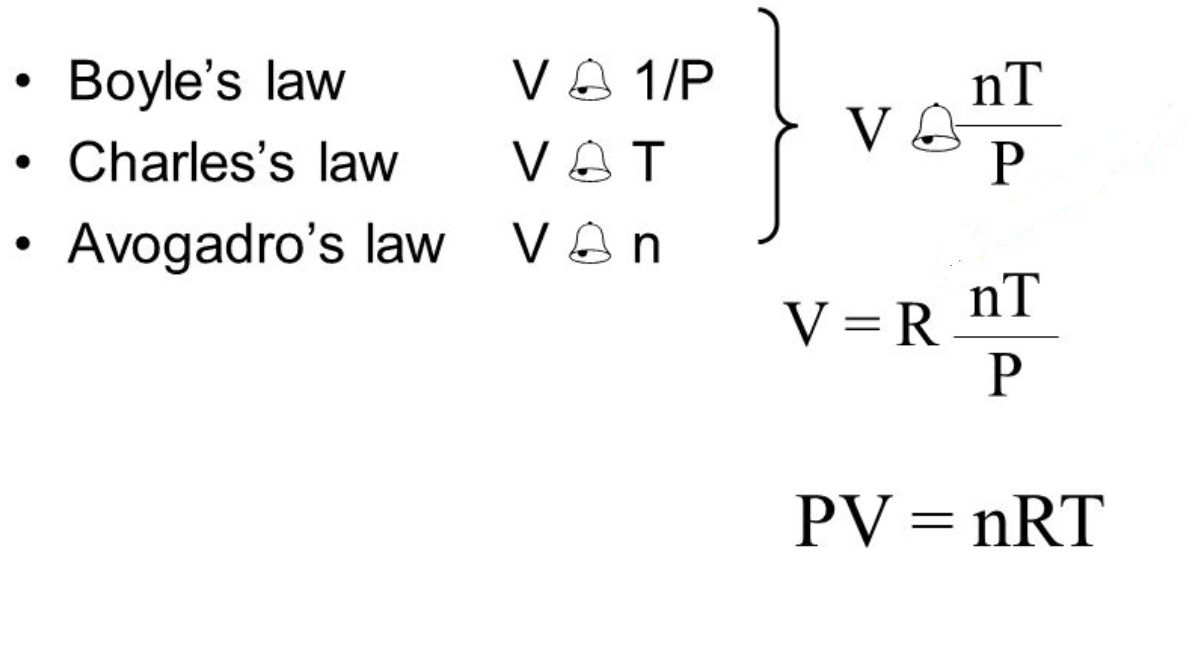
The ideal gas law PV nRT is applicable only ideal gases. Which of the following graphs correctly represents the relationship between the temperature and the. Ideal Gas Law PV nRT The pressure of a gas times its volume equals the number of moles of the gas times a constant R times the temperature of the gas.
Limitations of Ideal Gas Law. Start studying the The Ideal Gas Law Quiz flashcards containing study terms like Which laws can be combined to form the ideal gas law Avogadros law and Charless law describe a proportionality of the volume of a gas when the pressure is constant. Where P1V1 and T1 are the original values of the gas while P2V2 and T2 represent its final values.
The pressure volume and temperature of an ideal gas are related by a simple formula called the ideal gas law. The ideal gas law is a critical tool used in chemical. It relates the initial pressure volume and temperature of a gas to the final pressure volume and temperature after a change has taken place.
Tabsolute temperature measured in Kelvin. In Boyles Law PV k a constant. The ideal gas law is obtained by combining Boyles law Charless law and Avogadros law.
PV nT R PV nT. According to Avogadros law at constant temperature and pressure the volume of all. This relationship of direct proportion can be written as.
Using the Ideal Gas Law Experiment 1. The pressure and the temperature are constant. Charless Law Table 1.
P1V1 T1 P2V2 T2. The temperature of the ideal gas in the container shown is 300K. Charless law is an experimental gas law that describes how gases tend to expand when heated.
Where k Boltzman constant and N number of gas molecules. Feb 29 2016. The combined gas law then gives a simple and convenient formula that is used to solve gas law problems involving pressure.
Mathematically Ideal gas law is expressed as. The Ideal Gas Law Goal 2 Explain how the ideal gas equation can be constructed by combining Charless Boyles and Avogadros Laws and explain how the ideal gas equation can be used to derive each of the three two-variable laws. From there we can make several derivations.
Where V volume of gas. The ideal gas law is PV nRT where T is the absolute temperature and n is the number of moles of gas present in a system. The number of moles and the temperature are constant.
P₁V₁T₁ P₂V₂T₂ Ideal Gas Law. We can also use an equivalent equation given below. The gas occupies a V of 127 m3.
Where is the pressure of the gas is the volume taken up by the gas is the temperature of. Boyles law assumes that only pressure and volume changes.

The Ideal Gas Law Chemwiki Ideal Gas Law Teaching Chemistry Apologia Chemistry
Chapter 11 Gases The Gas Laws Of Boyle Charles And Avogadro Ppt Download
Comments
Post a Comment